Soft Palate and Its Motor Innervation: A Brief Review
Anatomy Physiology & Biochemistry International Journal Juniper Publishers
Abstract
Human soft palate plays an important role in upper airway functions such as speech, swallowing and respiration. However, neural control of the soft palate is poorly understood because innervation of this structure has long been controversial. In this review, the inconsistent and even contradictory observations regarding the motor innervation of the palatal muscles are summarized. We emphasize to use Sihler’s stain for documenting the nerves and their supply patterns within individual palatal muscles as studies have demonstrated that Sihler’s stain permits mapping of entire nerve supply within organs, skeletal muscles, mucosa, skin, and other structures. This wholemount nerve staining has unique advantage over other anatomical methods as all the nerves within the muscles processed with Sihler’s stain can be visualized in their 3-dimensional positions. Advanced knowledge of the neural organization of the soft palate is critical for a better understanding of its functions and for the development of novel neuromodulation therapies to treat soft palate-related upper airway disorders such as obstructive sleep apnea.
Keywords: Soft palate; Upper airway; Motor nerves; Innervation; Palatal muscles; Sihler’s stain
Abbreviations: IX: Glossopharyngeal Nerve; LVP: Levator Veli Palatine; MU: Musculus Uvula; OSA: Obstructive Sleep Apnea; PG: Palatoglossus; PP: Palatopharyngeus; TVP: Tensor Veli Palatine; V: Trigeminal Nerve; VII: Facial Nerve; X: Vagus Nerve; XI: Accessory Nerve; XII: Hypoglossal Nerve
Introduction
Soft palate and its involvement with obstructive sleep apnea (OSA)
The soft palate is the movable soft tissue that makes up the back of the roof of the mouth in mammals. The soft palate in humans plays an important role in speech, swallowing, and breathing. It has recently been drawing attention due to its involvement with OSA [1], affecting over 20 million Americans with very serious comorbidities [2]. OSA is caused by repetitive upper-airway obstruction during sleep involving primarily the tongue and the soft palate [3]. In patients with OSA, the upper airway muscles relaxed so much that the soft palate and surrounding tissues collapse and block the airway. In OSA, airway collapse in the velopharyngeal region is related to disturbances in the neural control of the soft palate caused by impaired sensory input, focal sensory nerve degeneration, and denervation of the palatal muscles [4]. Although the soft palate plays a vital role in life-maintaining functions and its dysfunction leads to life-threatening OSA, little is known about how it is controlled by nervous system to couple with its wide range of physiological behaviors and what pathologically induced changes occur in this structure. Poorly understood neural control of the soft palate is a potential obstacle to the development of novel therapies for OSA and other related disorders.
Controversies regarding motor innervation of soft palate
The shape, position, and movements of the soft palate are maintained by five pairs of muscles, including tensor veli palatini (TVP), levator veli palatini (LVP), palatopharyngeus (PP), palatoglossus (PG), and musculus uvula (MU) (Figure 1). However, little is known about how the palatal muscles work during various motor tasks and how these muscles and innervating nerves are organized within the soft palate. This is largely due to a gap in our knowledge of the neuromuscular specializations of the soft palate.
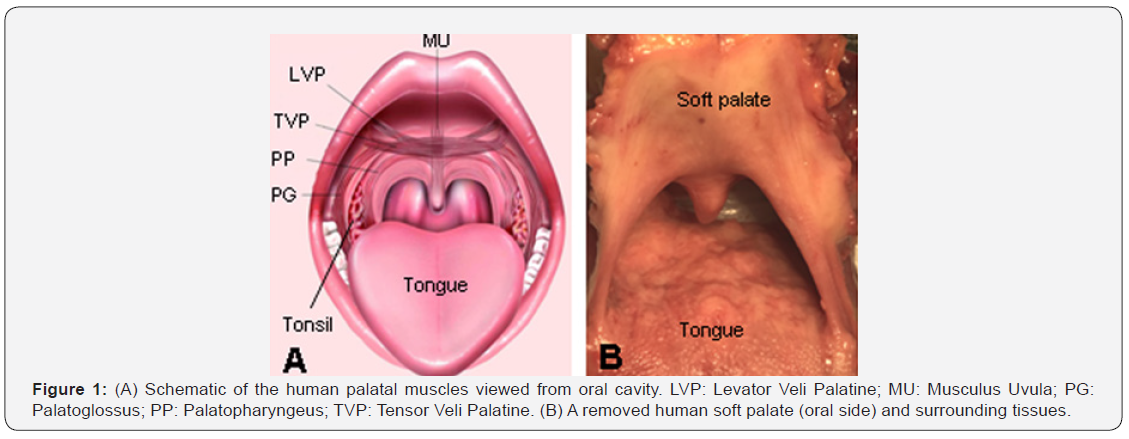
At present, neural control of the soft palate and its surrounding structures is poorly understood because innervation of this region has long been controversial. It is generally described that the palatal muscles are innervated by the pharyngeal branch of the vagus nerve (X), except for the TVP muscle, which is supplied by the trigeminal nerve (V) [5]. However, velopharyngeal movements were observed by stimulation not only to the X, but also to the glossopharyngeal nerve (IX) and facial nerve (VII) [6]. Anatomic and physiological studies showed that the LVP receives its motor innervation from the IX nerve [7], lesser palatine nerve [8], or VII nerve [9].
In contrast, retrograde axonal tracing studies in cats identified LVP motoneurons on both sides (bilateral innervation) in the nucleus ambiguus, but not in the facial nucleus [10,11]. PP muscle was reported to receive innervation from the pharyngeal branch of the X nerve [5,12], the X and XI nerves [13], or from the pharyngeal plexus and VII nerve [8]. Some investigators reported that the PG was innervated by the IX, X, XI [14], XII [15], or by the X, but not by the XII or IX [16]. Whereas others found that the intra-lingual and extra-lingual PG received their innervation from the XII nerve and the X nerve, respectively [17]. It is generally described that the IX provides motor innervation only to the stylopharyngeus muscle [5]. However, several studies showed that the IX also contribute motor innervation to the palatal muscles in some animals [7,18]. Clearly, the innervation source of the soft palate has not yet been fully clarified. The inconsistent and even contradictory findings regarding the innervation of the soft palate may be attributed at least in part to the fact that previous studies failed to demonstrate the precise distribution of the individual nerves. It is difficult to explain why different findings were obtained from the same species by different investigators and by different methods. One facet may be the fact that the neuromuscular organization within the soft palate is complicated, and that a specific nerve branch innervating a given muscle might be wrongly identified by gross dissection. Therefore, precise information regarding the innervation of the soft palate is lacking.
Entire nerve mapping with Sihler’s stain
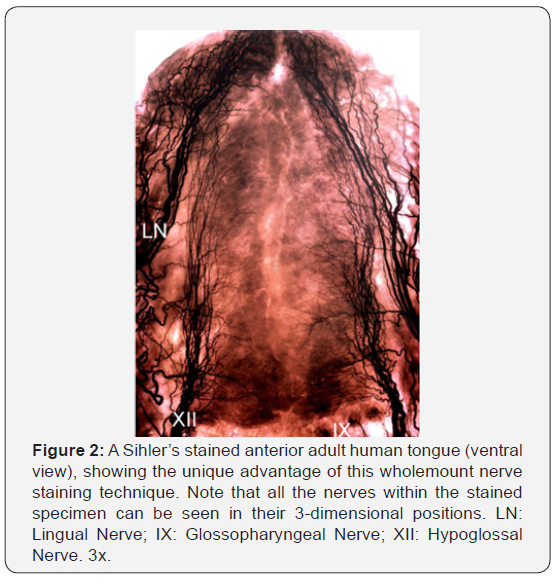
A recent advance in anatomical investigations is the use of Sihler’s stain, a wholemount nerve-staining technique that clears soft tissue while counterstaining all nerves [19]. This technique permits mapping of entire nerve supply patterns of organs, skeletal muscles, mucosa, skin, and other structures. The unique advantage of Sihler’s stain over other anatomical methods is that all the nerves within the stained specimen can be seen in their 3-dimensional positions (Figure 2). Therefore, the distribution of all nerves can be determined by tracing them from the origins to the terminations. Sihler’s stain has been extensively used in our laboratory to study innervation of the larynx [20], pharynx [21], and tongue [22]. Using this technique, we have clarified some controversies regarding the innervation of these structures, uncovered some previously unknown anatomical facts, and yielded many new discoveries. We were the first to map out the entire pharyngeal plexus and precise distribution of the individual nerves in humans using Sihler’s stain [21]. One of our important discoveries was that the pharyngeal branch of the IX nerve also innervates the pharyngeal constrictor muscles [21]. This is contrary to the prevailing view that the IX only provides motor innervation to the stylopharyngeus muscle [5]. Surprisingly, the innervation of the soft palate has never been studied using Sihler’s stain in any mammal. Further studies are warranted to document nerve supply patterns of the soft palate.
Clinical significance
Details regarding the neuroanatomy of the soft palate are critical for the development of novel neuromodulation therapies to treat OSA. The most common treatment for OSA, continuous positive airway pressure, has been associated with poor patient compliance. Despite a number of surgical methods being available to treat OSA, the results are discouraging [23-26], with only about 50–65% of patients gaining benefit from these procedures [27]. Hypoglossal nerve stimulation has been used to treat OSA [14,28]; however, hypoglossal nerve stimulation may be applicable to only certain OSA patients as this therapy usually produces a partial response [28]. A significant percentage of OSA patients do not respond to hypoglossal nerve stimulation. Most of these “nonresponders” appear to have their airway obstruction caused by the soft palate. We hypothesize that some severe OSA patients “non-responsive” to hypoglossal nerve stimulation could be treated by electrical stimulation of the motor nerve(s) innervating the soft palate.
Conclusion
There is a pressing need to determine the innervation of the soft palate using reliable approaches such as Sihler’s stain. Clarifying the controversies concerning the innervation source and distribution of the nerves supplying the soft palate would be useful for clinicians, surgeons, and academics who manipulate and keep particular interest for this anatomically complex and functionally important structure. Advanced knowledge of the neural organization within soft palate is helpful for better understanding its functions, performing electrophysiological testing, and developing novel neuromodulation therapies to treat OSA.
To Know More About Anatomy Physiology & Biochemistry International Journal Please click on:
For more Open Access Journals in Juniper Publishers please click on:


Comments
Post a Comment