Study of the Antioxidant Activities of Avocado (Persea Americana Mill.) and Three Banana (Musa Paradisiac L.) varieties by FRAP and Rancimat Assays
Anatomy Physiology & Biochemistry International Journal Juniper Publishers
Abstract
The antioxidant activity of three banana (Musa paradisiaca L.) fruit varieties (Robusta, William’s-2 and Dwarf Cavendish), obtained from Woramit Horticultural Site, and avocado (Persea Americana Mill.), purchased from fruit shops in Bahir Dar City, have been studied by ferric reducing antioxidant power (FRAP) and Rancimat Materials and Methods. FRAP values of the fruits are expressed as ascorbic acid equivalent antioxidant capacity (AEAC) in μg AA/g of fruit weight and the Rancimat values are expressed in terms of induction index (II). The FRAP values of the fruits were 8.43±0.145, 7.46±1.14, 6.66±0.04 and 6.27±0.898 and Rancimat result were 1.076, 1.025, 1.041 and 1.046 for avocado, Robusta, William’s-2 and Dwarf Cavendish, respectively. Avocado fruit extract showed relatively higher antioxidant activity than the banana fruit varieties.
Keywords: Biomarkers; Optimal training; Internal loads; Runners
Abbrevations: ROS: Reactive Oxygen Species; AOC: Antioxidant Capacity; FRAP: Ferric Reducing Antioxidant Power; TCA: Tri-Chloro Acetic Acid; AEAC: Ascorbic Acid Equivalent Antioxidant Capacity; II: Induction Index; RP: Reducing Power; IP: Induction period
Introduction
Epidemiological studies have provided evidence of an inverse correlation between diets rich in fruits and vegetables and free radical induced diseases [1]. The protection that fruits and vegetables provide against those diseases has been attributed to the various antioxidants contained in them [2]. The antioxidants produced by plants to control the oxidative stress caused by sunlight and oxygen also serve the same purpose in the human body [3]. The richest source of antioxidant compounds are fruits, vegetables and medicinal herbs [1]. Scientific evidence accumulated over the years indicates that free radicals cause oxidative damage to lipids, proteins and nucleic acid. They may lie at the heart of diseases including cancer and atherosclerosis [2]. The body is continually exposed to free radicals whether they are produced via endogenous biochemical processes or as a result of external lifestyle factors [4]. A free radical may be defined as a molecule or a molecular fragment that is capable of independent existence and containing one/more unpaired electrons in its outermost atomic/molecular orbital [5]. Generation of free radicals or reactive oxygen species (ROS) during metabolism and other activities beyond the antioxidant capacity (AOC) of a biological system gives rise to oxidative stress [6]. It is an imbalance between ROS and defence and repair antioxidant systems [5]. To lessen and ultimately prevent the damaging effects of free radicals, the responsibility lies with antioxidants. They are substances that when present at low concentrations compared with those oxidizable substrate significantly delays or inhibits oxidation of that substrate [6-8]. The roles of antioxidants are to neutralize the excess free radicals, to protect the cells against their toxic effects and to contribute to disease prevention [9]. One of their the protective mechanisms is their ability to scavenge free radicals in the human body and thereby decrease the amount of free radical damage to biological molecules [1].
Avocado (Persea Americana Mill. family of Lauraceae) is an important oleaginous fruit [10] and is native fruit of Mexico and Central America [11]. Its health benefit is may be due to its content of over 20 essential nutrients and various potentially cancer-preventing phytochemicals [12]. It contains numerous antioxidant phytochemicals such as persin, persenones A and B [13] vitamins E and C [13,14], p-coumarylquinic acid, catechin, caffeic acid, p-coumaric acid, leucoanthocyanidin, isoflavone, and epicatechin [15,16]. Banana (Musa paradisiaca L.) is one of the tropical fruits [17] and its origin is placed in South-east Asia, in the jungles of Malaysia, Indonesia and Philippines [18] and some varieties are found to be genetically linked with some species from Africa [19]. Banana pulp has been reported as having various antioxidants such as gallocatechin [20], phenolic compounds, flavanoids, β-carotene, ascorbic acid [19] and dopamine [17,19]. It contains phenolic acids such as gentisic acid, caffeic acid, ferulic acid, cinnamic acid, protocatechuic acid, and (+)-catechin [21].
epicatechin [15,16]. Banana (Musa paradisiaca L.) is one of the tropical fruits [17] and its origin is placed in South-east Asia, in the jungles of Malaysia, Indonesia and Philippines [18] and some varieties are found to be genetically linked with some species from Africa [19]. Banana pulp has been reported as having various antioxidants such as gallocatechin [20], phenolic compounds, flavanoids, β-carotene, ascorbic acid [19] and dopamine [17,19]. It contains phenolic acids such as gentisic acid, caffeic acid, ferulic acid, cinnamic acid, protocatechuic acid, and (+)-catechin [21].
It is of a great interest to consumers and nutritionists to quantify the antioxidant properties of various foods because they are clearly important to human life [22]. Among various plants, fruits are considered rich in antioxidants due to the presence of compounds like polyphenolics and vitamins, which play an important role in scavenging free radicals. Therefore, it is of interest to study the antioxidant activities of a fruits. Thus, the purpose of this study is to evaluate and compare in vitro the antioxidant activities of banana (Musa paradisiaca L.) and avocado (Persea Americana Mill.) fruits. The antioxidant activities are measured using ferric reducing antioxidant power (FRAP) and Rancimat assays.
Materials and Methods
Fruits
The three banana fruit varieties were brought from Woramit Horticultural Site, Amhara Region, North-West Ethiopia. The banana fruit varieties studied were Dwarf Cavendish, William’s -2 and Robusta. The avocado fruit were purchased from a fruit shop in Bahir Dar City. All fruits were kept in carton for ripeness.
Chemicals and reagents used
All chemicals and reagents used in this work were analytical grade reagent and were used with no further purification. Trichloro acetic acid (TCA), ferric chloride (FeCl3), ascorbic acid, potassium hexacyanoferrate [K3Fe(CN)6], sodium phosphate dibasic (Na2HPO4) and sodium phosphate monobasic dihydrate (NaH2PO4.2H2O) were all obtained from Brulux, India. Distilled water was used throughout the work.
Sample Preparation
Ferric Reducing Antioxidant Power (FRAP) assay: All fruits were washed with tap water to remove dusts and were peeled off with knife. The peeled fruits (edible portions) were cut into pieces and homogenized. Then, 40g of each fruit was mixed with 200mL of distilled water. The mixture was transferred to the grinder and was blended for one minute. The resulting juice was shaken manually for 10minutes to increase solubility. The juice was filtered by sieves to separate the particulate. The non-particulate juice was filtered by Whatman No.1 filter paper and was centrifuged at 3000rpm for 10minute to get clear supernatant. The clear supernatant solution was used for the analysis [24].
Rancimat Assay: The edible portion of each fruit were cut into pieces and homogenized and was oven-dried at 80°C up to 2 days until constant weight was achieved [23]. Then, the resulting dried sample was powdered by blender and the powder was mixed with edible (sunflower) oil at 5% concentration and the mixture was used for analysis.
Determination of antioxidant activity by FRAP Assay
The ferric reducing power of the fruit extracts was determined by using potassium ferricyanide-ferric chloride method [24]. According to this method the reduction of Fe3+ to Fe2+ was determined by measuring absorbance of the Prussian blue complex. Different dilutions of the extracts (1.25, 2.5, 5.0 and 10%v/v) amounting to 1mL were added to 2.5mL of 0.2M phosphate buffer (pH 6.6) and 2.5mL potassium ferricyanide (1%) complex. The mixtures were incubated at 50oC for 20min. The reaction was stopped by adding 2.5mL of trichloroacetic acid (TCA) solution (10%) and then centrifuged at 3000rpm for 10min. To 2.5 mL portion of the mixture, 2.5mL distilled water and 0.5mL of 1% freshly prepared FeCl3 solution was added and the absorbance was measured by using UV-Visible spectrophotometer at 700nm. Ascorbic acid used as a standard was run similarly and the reducing power of the fruit extracts was compared with ascorbic acid. A graph of absorbance versus fruit extract concentration was plotted to observe the reducing power [24,25]. The iron (III) reducing activity determination was expressed in μg ascorbic acid equivalents/g of fruit weight as given by the formula
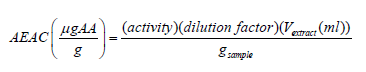
Where AEAC = ascorbic acid equivalent antioxidant capacity; μg AA = microgram of ascorbic acid and activity can be calculated from calibration curve of the equation y = ax+ c as given by the following equation:
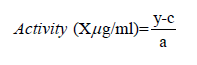
Where: y = absorbance of the sample
c = y-intercept and
a = slope
The percentage of reducing power (%RP) of the sample as compared to standard (ascorbic acid) was calculated by using the formula [26]:
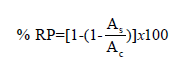
Where %RP= Percent of reducing power, Ac = absorbance of standard (ascorbic acid) at maximum concentration tested and AS = absorbance of the sample.
Determination of antioxidant activity by rancimat assay
The 3g of the sample (mixture) was weighed into a reaction vessel, placed in a heating block kept at 120oC, with airflow of 20L/h. During the oxidation process volatile compounds were released and collected in the receiving flask filled with distilled water, and then their conductivity was measured and recorded. Results of the experiments were automatically evaluated by the Rancimat software. The completion of the induction period (IP) was indicated with a sudden rise in conductivity of water due to the dissociation of volatile carboxylic acids. Sunflower oil without antioxidant as the control was run similarly. On the basis of obtained IP readings, antioxidant efficiency (activity) of additives was established. The induction index (II) was determined as the ratio of the induction period of a sample with antioxidant to the induction period of the control [27,28]. The results of the fruits were expressed in terms of induction index. It was calculated by the following equation:
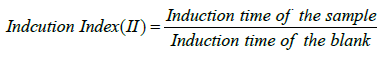
As it was observed from the formula, a higher induction index indicates higher antioxidant activity [28].
Result and Discussion
Construction of calibration curve
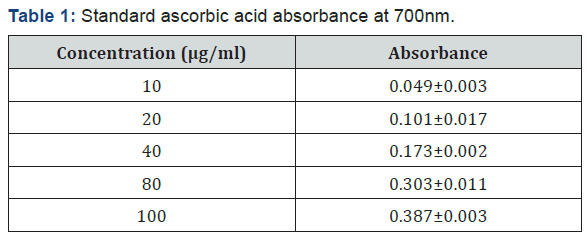
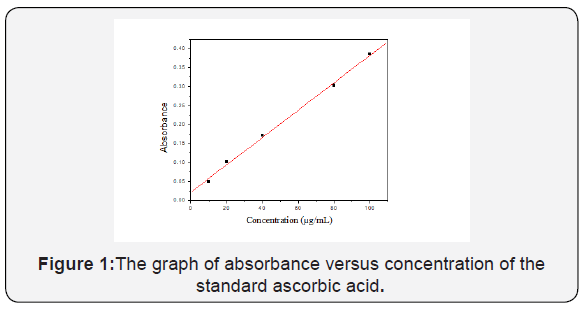
The calibration curve was constructed by using ascorbic acid standard. After preparation of different concentration (10,20,40,80 and 100μg/mL) of ascorbic acid standard the absorbance of the solutions was measured at 700 nm. The resulting absorbance was shown in Table 1. The equation of the calibration curve was obtained from the resulting absorbance versus concentration of the graph and the equation was,
y = 0.00362x + 0.0212 ,
R2 =0.998.
Figure 1 shows the graph of absorbance versus concentration of the standard ascorbic acid, R2 =0.998.
FRAP assay
FRAP is a method based on a single electron transfer reaction between an oxidant and antioxidant, i.e., antioxidants are oxidized by the oxidant Fe (III). As a result a single electron is transferred from the antioxidant molecule to the oxidant [29]. The reducing capacity of fruits may serve as a significant indicator of its potential antioxidant activity. After mixing of fruit extracts and ferric ion complex in the presence of phosphate buffer and incubation at 50oC for 20 minutes, the yellow color of the ferricynanide complex is changed. The observed color change is likely to be due to the reduction of ferric ion to ferrous ion and this occurs most probably due to the presence of reducing agents (antioxidants) in the fruits and fruit extracts. These reducing agents (antioxidants) are likely water-soluble antioxidants, like ascorbic acid, phenolic acids, polyphenols, etc which are found in those fruits and fruit extracts. The product was visualized upon addition of free Fe3+ ions after the reduction reaction, by forming the Prussian blue colour complex, (Fe3+)4[Fe2+(CN-)6]3 as shown below by chemical equation, which was the confirmatory test for ferrous ion and quantified as absorbance measurement at 700 nm.
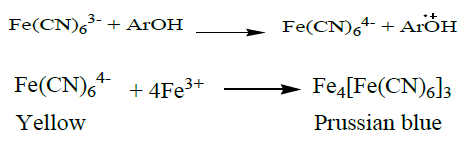
Where, ArOH represents an antioxidant
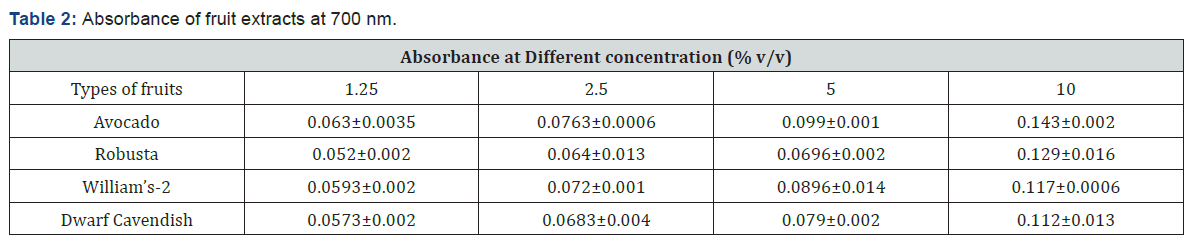
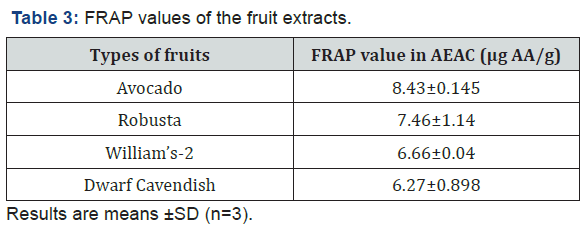
The color change develops for all fruit types, but there was difference in the intensity of the color which develops. The difference may be linked to the difference in the antioxidant constituent of the fruits and the concentration of the extracts. Table 2 shows the average values of the absorbance of the fruit extracts at different concentration. The absorbance of each fruit was not the same at all concentration and this was due to the difference in the antioxidant composition of the fruits. The reducing power of the fruits was given graphically in terms of concentration versus absorbance. As shown in Figure 2, increased absorbance as concentration increases was an induction of higher reducing power and antioxidant capacity as concentration increases and the sudden rise in the absorbance of Robusta as we go from 5% to 10% v/v concentration needs further investigation. In this assay, increased absorbance indicated increased reducing power. The reducing power (antioxidant activity) of all those tested fruits increased in a concentration-dependent manner as shown in Figure 2, but it is not in a constant manner and this may be due to the difference in the antioxidant’s composition of the fruits. For instance, at 10 %v/v concentration, reducing power of avocado showed better activities than that of banana varieties. These results suggest that avocado have a remarkable potency to donate electron to reactive free radicals, converting them into more stable non-reactive species and terminating the free radical chain reaction. The FRAP values of the fruit extracts was expressed in terms of AEAC (ascorbic acid equivalent antioxidant capacity). The results were expressed as μg of ascorbic acid (AA) equivalent per 1g, i.e. the quantity of AA required to produce the reducing power as the extract in 1g of the edible portion of the fruit. As shown in Table 3, avocado have higher FRAP value than the banana varieties and among the banana fruit varieties, Robusta have higher FRAP value followed by William’s-2 and Dwarf Cavendish.
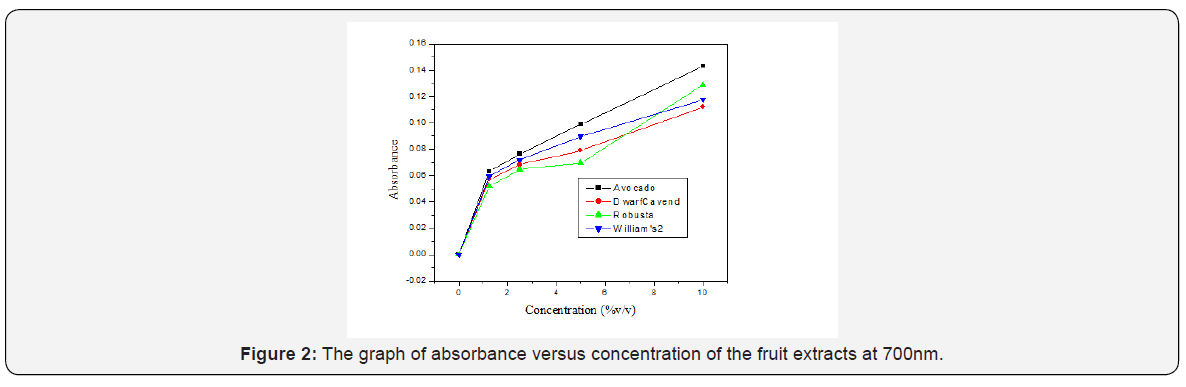
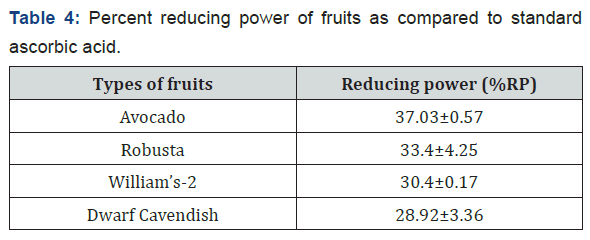
The %RP of the fruit extracts as compared with ascorbic acid standard was calculated at 10% v/v concentration of the fruit extracts. The %RP of avocado was higher when compared to the banana varieties i.e. it has 37.03% reducing power when compared with the standard ascorbic acid at maximum tested concentration and followed by Robusta, William’s-2 and Dwarf Cavendish. Table 4 gives the %RP of the fruit extracts at 10 % v/v.
Rancimat assay
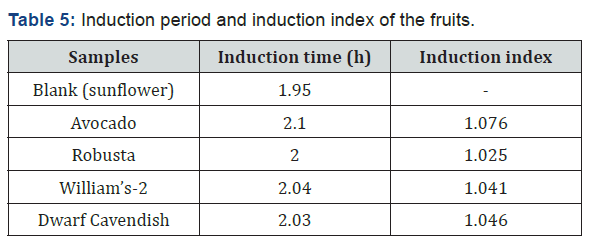
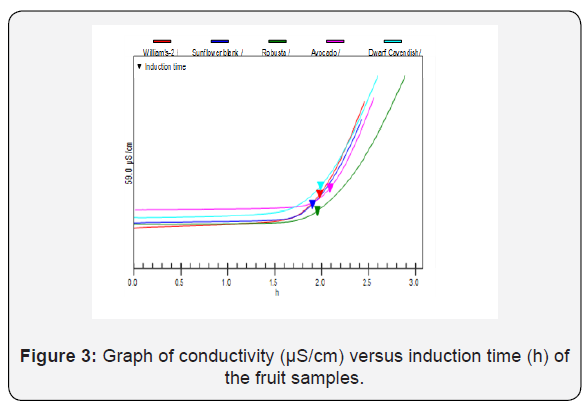
The Rancimat method is an automated version of the active oxygen method for the determination of induction time, the socalled stability time of food products. The result obtained shows that the IP in the presence of the fruit sample was higher than that of the blank, i.e. the fruit samples increase the IP of the oil that means they inhibit the oxidation of the oil. Thus, the increase in the IP of the oil which was mixed with the fruit extract was an indicative for the presence of antioxidant compounds in the fruit. Those antioxidant compounds were most probably lipid soluble antioxidant compounds such as vitamin K, carotenoids, etc. and the AOA of the fruit was expressed in terms of the induction index. The fruits showed AOA by this method (II=1.076-1.046), but there was no significant difference between the results. The induction index of the fruits was calculated, and Table 5 gives the induction time and induction index of the fruits. The higher induction index is the indication of the better antioxidant activities of that fruit extract [25]. AOA as determined with the Rancimat method decreases in the order of avocado, Dwarf Cavendish, William’s-2 and Robusta. Accordingly, avocado fruit has higher AOA than the banana fruit varieties as indicated in Figure 3.
Conclusion
The fruits tested have the power to reduce ferric ion to ferrous ion which was confirmed by the Prussian blue color which was formed up on addition of ferric chloride to the mixture. The fruits tested also increased the induction period of sunflower oil which suggests that they have the power to inhibit the autoxidation of oils by scavenging the free radicals which are formed during oxidation of oils at high temperature. Thus, by both the FRAP and Rancimat assays, the avocado and banana fruits studied do possess antioxidant components that are beneficial for health. The antioxidant composition of fruits is reported to be affected by the geographical origin and harvest time [7]. That avocado fruits have shown better antioxidant property than the banana fruit varieties considered in this study is in agreement with literature reports [30]. In general, since avocado and banana fruits considered in this study have antioxidant activity, their inclusion in our diets is a viable way of protecting our bodies from free radical-induced diseases.
To Know More About Anatomy Physiology & Biochemistry International Journal Please click on:
For more Open Access Journals in Juniper Publishers please click on:


Comments
Post a Comment